DENTAL
PATHOLOGY AND DIET: SECOND THOUGHTS
In Otte, Marcel 1995 Nature et Culture,
Actes du Colloque international de Liège, December,
1993
Etudes et Recherches Archéologiques de
l'Université de Liège. no 68: 457-480.
M. JACKES and D. LUBELL
Department of Anthropology
University of Alberta
Edmonton, Alberta, Canada T6G 2H4
Abstract
Dietary regimes have a strong bearing on dental pathology rates. In
this paper we use data from large samples of Portuguese Mesolithic and
Neolithic dentitions to show that caries rates are not, however, easy
to establish. We demonstrate that while comparable methods and samples
are obviously necessary, there are more important considerations which
have not been discussed in the literature. Accurate observation of
different types of caries will be affected by both the type of burial
and depositional conditions. Furthermore, it is necessary to take into
consideration factors which may bias the available sample, e.g., the
demography of the population and differential preservation of tooth
classes and of specific age categories. In sum, not only burial
practices, but mortality rates, the nature of the burial deposits, the
methods of excavation and curation, and the techniques of observing and
recording caries, will all introduce variations into caries rates, as
well as the immediate biological determinants of oral health. We
conclude that it may be impossible to obtain accurate dental pathology
rates, and that variations in pathology cannot be attributed to diet
alone.
Introduction
We have recently published a detailed study on dietary change from
the Mesolithic into the Neolithic, based on large skeletal collections
from several Portuguese sites including Moita do Sebastião,
Cabeço da Arruda and Casa da Moura. Using stable isotope
analyses, together with discussions on rates and types of attrition, we
have been able to follow changes from 8,000 BP to 4,000 BP (calibrated
years). While dental pathology is included in our discussions, we show
that pathology does not give us the clear and simple picture one might
expect (Lubell et al. 1994).
Yet caries rates are considered important indications of diet, and
there has long been a certainty that alterations in caries rates mark
dietary shifts world wide.
Part of the variability that makes the picture unexpectedly fuzzy
will be the result of unknown dietary factors. But non-dietary factors
also come into play. Two considerations are important here.
a) The first depends on our ability to observe and recognize caries
as the type of caries changes through time, and as burial practices
change through time.
b) The second relates to problems of sample bias due to demographic
and taphonomical factors.
Problem 1
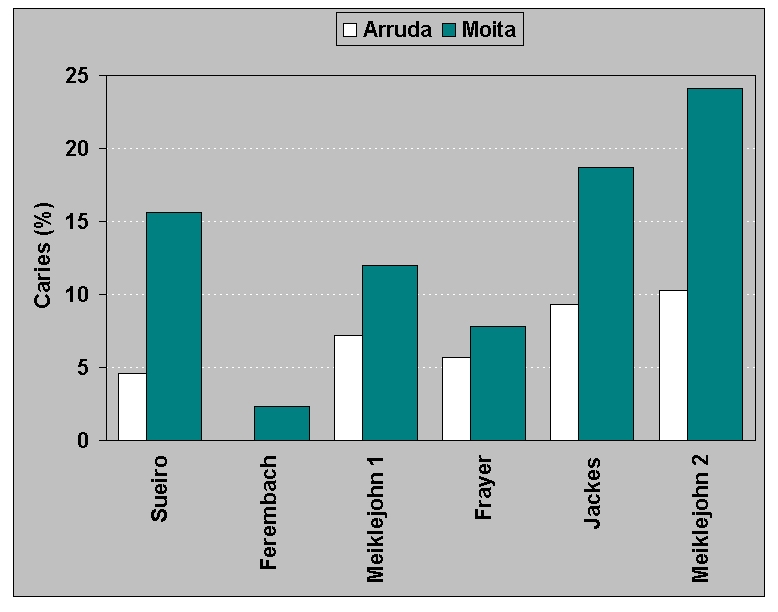
Analyses of Portuguese Mesolithic dental pathology (Figure
1) demonstrate that it is difficult to identify the "real caries
rate". Some of the variation can be explained by different criteria for
the definition of caries, and some by different or biased samples. For
example, the sample used by Frayer (1987) was less than 50% of the
teeth from Cabeço da Arruda and excluded some of the oldest
individuals. The only real consistency is the contrast between the two
Mesolithic sites, Moita do Sebastião and Cabeço da
Arruda. Although they are only 3 km apart, Moita, with a mean date 300
years earlier, has a higher caries rate than Arruda.
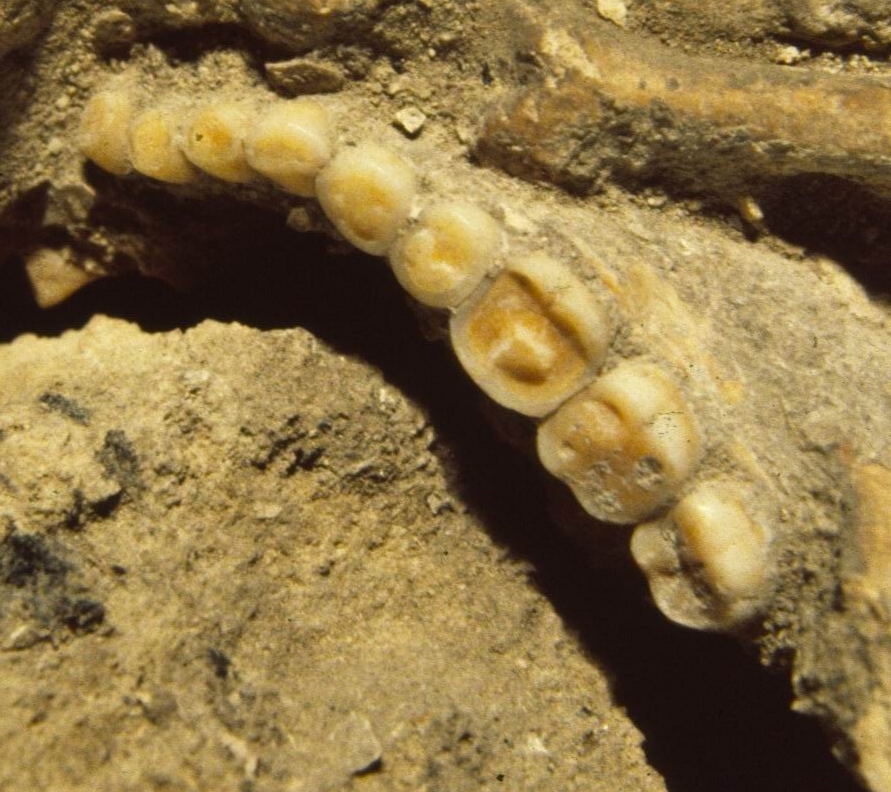
Errors in observation of Portuguese Mesolithic caries are to be
expected and rates may vary for the simple reason that heavy matrix
obscures the fossils (Figure 2). Our
results are based on multiple observations (by four individuals, and
with progressively more complete cleaning, over a six year period) of
dentitions preserved in the Geological Survey of Portugal in Lisbon. We
have also made some observations of dentitions from these sites which
are housed in the Institute of Anthropology in Porto, but because the
material there is not cleaned we will not use those data here.
The state of preservation and degree of cleaning becomes
particularly important when interproximal caries are frequent. Complete
dentitions set in their alveoli cannot be examined fully, for it is
impossible to observe all interproximal surfaces, and the smallest
amount of matrix compounds the problem. In such a situation rates must
be reported in very specific ways. For example, we would report, not
general caries rates, but specifically that Moita has a higher
mandibular molar occlusal caries rate than Arruda (14% of lower molar
occlusal surfaces at Moita vs. 7% for Arruda), even despite the
slightly higher rate of attrition in Moita molars.
We interpret the high Mesolithic caries rates as a result of
consumption of sticky fruits, for example, dried figs. However,
consumption of sticky fruits might be expected to increase the rate of
interproximal caries; but we find that the ratio of interproximal to
occlusal caries increases through time (Figure
3). Perhaps our interpretation with regard to fruits may not be
correct, or perhaps we are unable to see all interproximal caries in
the Moita dentitions.
The question of interproximal caries is of increased importance in
the comparison of Mesolithic and Neolithic pathology rates, and this
raises the question of burial practices and depositional conditions.
Mesolithic Portuguese dentitions are generally in situ in the
bone, while Neolithic dentitions rarely are retained in the alveoli.
This is due, in large part, to replacement of the Mesolithic pattern of
individual burial by ossuary burial in the Neolithic. Most Neolithic
teeth are found loose.
Our sample from Casa da Moura included about 5,000 loose teeth, and
we were therefore able to do an extremely detailed study, examining
every tooth surface using a 10x loup or a dissecting microscope. As a
result, we can specify the number of unequivocal caries and state, with
complete confidence, when toothpicks were used to relieve the
discomfort of caries (Figure 4a).
However, the presence of root caries in the Casa da Moura sample
prevents us from being able to state confidently the caries rate for
this population. The problem relates to caries such as the one in
Figure 4b at the cemento-enamel junction
(CEJ)
of an upper molar.
When teeth are retained into old age, root surfaces are exposed. Our
data from Mesolithic and Neolithic dentitions indicate that over 3 mm
of root was laid bare by the time secondary dentin was exposed. Modern
clinical research shows that under such conditions one can expect root
caries, especially when the diet is based on carbohydrates with little
sugar consumption (Fure and Zickert 1990); and we hypothesize increased
dependence on cereals and reduced dependence on dried fruits in many
Portuguese Neolithic sites. Adults, and even young children, had high
rates of CEJ caries from the introduction of agriculture and throughout
the period before sugar was widely available in Europe (O'Sullivan et
al. 1993).
Unfortunately, there is post-mortem erosion at the CEJ in many
teeth; for example, at least 15% of upper molars have marked erosion.
We interpret this cemento-enamel junction erosion as postmortem damage
(Figure
4c, d, e). If
the Casa da Moura CEJ furrowing is in
vivo and pathological, there should be significant age trends in
CEJ furrowing on the roots of loose teeth. We have not found a
significant relationship between age indicators and the presence of CEJ
erosion in any tooth class. Thus, not all the CEJ erosion can be
attributed to pathology. There may be a generalized relationship
between broad wear categories and CEJ erosion, indicating that teeth of
older individuals are more susceptible to postmortem damage and also,
perhaps, that active caries foci at time of death predispose the tooth
root to postmortem damage because of demineralization of the cementum
and exposure of the underlying less mineralized dentin.
Thus we have two problems: the first, that root
caries may be both mimicked by and masked by postmortem destruction;
and the second, that root caries at the approximal CEJ locations are
likely to be common and underreported in the literature. At least a
third of all Casa da Moura mandibular molar interproximal caries are,
or originated as, root caries at the CEJ. This is in perfect agreeement
with rates reported by O'Sullivan et al. (1993: 150) for
pre-17th century archaeological samples from Britain. Had the Casa da
Moura teeth been retained in their sockets, or had matrix similar to
the concreted Mesolithic midden matrix been present, the majority of
these caries would not have been observed.
Problem 2
Now we would like to turn to Problem 2: the other reason why we can
never be sure of the "real caries rate". Taphonomy is normally
considered to be the province of palaeontologists. But it has an
aspect, differential diagenesis, which although of minimal concern to
faunal analysts, must be considered by the human osteologist. Given
purposeful human burial, human osteologists need rarely worry about how
bone accumulations came to be where they are. But we must be concerned
about bone preservation and how it biases our studies - the effect of
differential diagenesis on the analysis of cemetery populations.
This aspect of taphonomy has been largely ignored even though Masset
(1973) urged its importance 20 years ago. Belgian work (Toussaint 1991)
on bone distribution and body part representation is in the vanguard in
Europe, but we have to consider, in addition to the preservation of
elements, the age-at-death factor within the preserved elements.
When we try to understand the interaction of the human organism with
the environment and the mediation of culture in this interaction, we
normally analyze age-dependent characteristics, of which dental
pathology is a very important example. In order to understand the
dental pathology rate we must also take into account (a) the age
distribution of the dead and (b) the possibility that the bones of
young adults are preferentially preserved.
We have an extremely clear example of the first possibility in a
site in Ontario with extraordinarily high mortality because of
epidemics, famine and warfare (Jackes 1988). Analyses of stable
isotopes and trace elements have shown that the maize component in the
diet was very high (see also Schwarcz et al. 1985) and Jackes
(1988) suggests that the caries rates (which are about half the
expected rates) are determined not only by diet but by the fact that
average age at death was very young. Thus, it is worth repeating that if
dental pathology is age-dependent and we do not have a clear idea of
the age distribution of our samples, it is meaningless to say that site
A has 20% pathology and site B has 40% pathology. We may really be
saying that at Site A there are very few old people, while the Site B
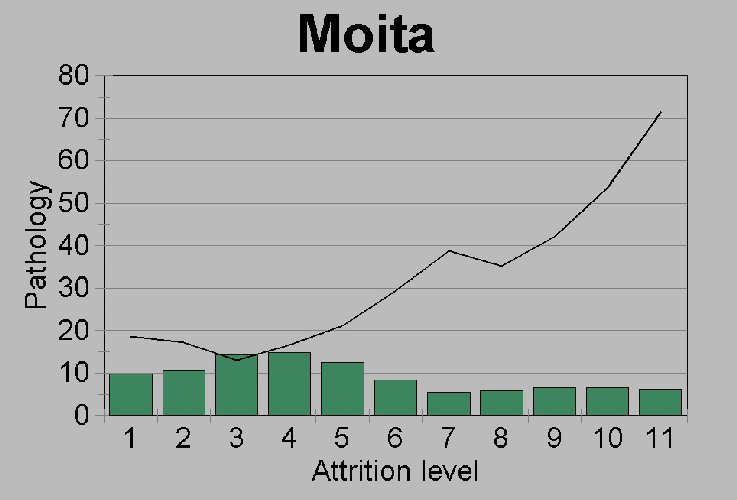
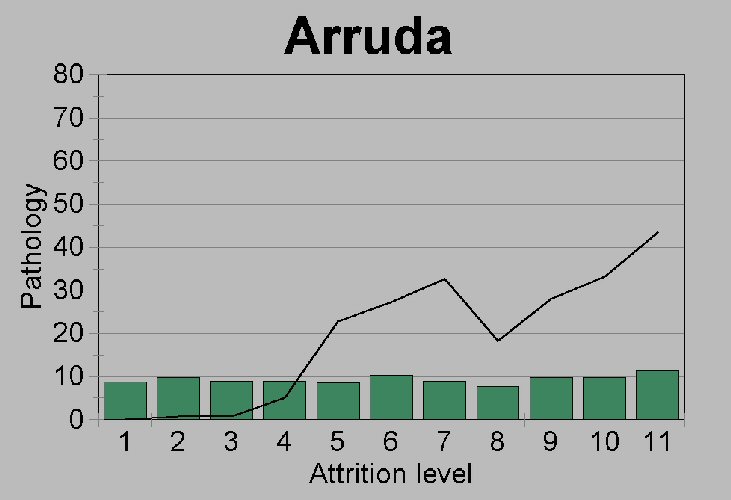
sample has relatively more old people. Our reason for maintaining that
the difference between Moita and Arruda caries rates is real, despite
questions of lowered attrition rates and possible differences in
age-at-death distributions, is that both sites show a fairly unbiased
sample across all attrition levels (Figure 5a,b,c).
The second factor which may confound osteological studies is the
faster decomposition of the skeletons of the elderly. A 75 year old has
about 30% less bone than a young adult of 25. While endosteal
resorption accounts for much bone loss in the elderly, intracortical
porosity also contributes. There are more Haversian canals, the canals
are bigger, there are over 200% more resorption spaces and those are
filled with new bone more slowly in the old than in the young (Mazess
1983; Martin and Burr 1989). The thinner cortex and greater porosity of
the compactum of the old, together with the reduction of the number of
trabeculae in spongey bone, allow bones to break more easily under soil
pressure and to be more subject to microbial action. Furthermore,
cortical bone with more spaces is more likely to be subject to
microbial action (Jackes 1990; Jackes et al. 1992, 2001).
Thus the bones of the old are preferentially comminuted or
destroyed.
As a test of the effect of this problem, we will examine whether
dental pathology rates might be incorrect as a result of differential
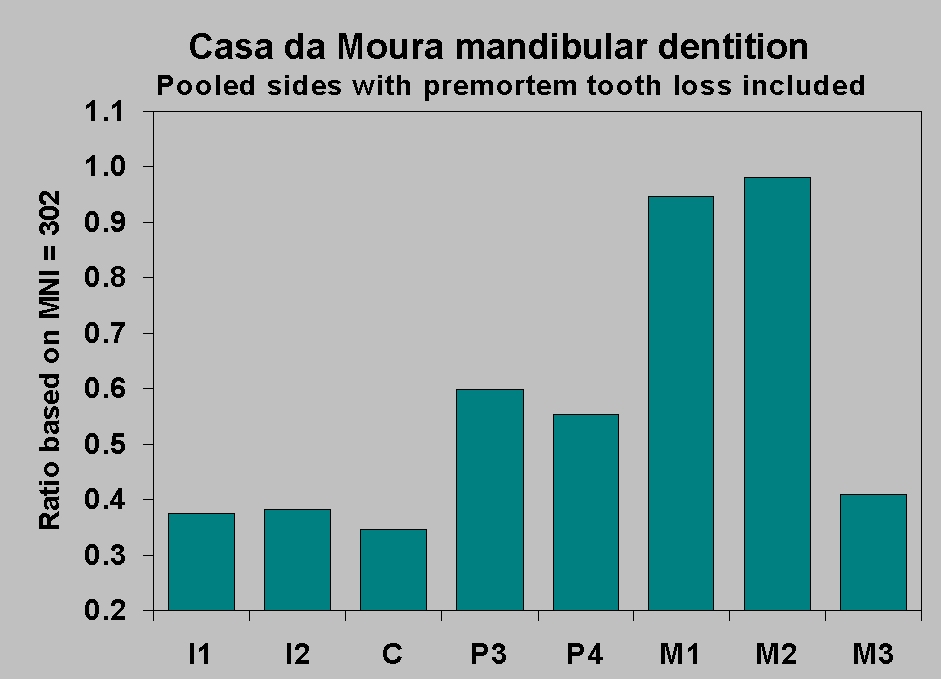
representation of parts and age classes. Our data are the more than
5000 teeth from Casa da Moura only one third of which are still in
situ in alveolar bone. We probably do not have exact figures here
- the material was excavated in the mid-nineteenth century (Delgado
1867) and recent re-excavation demonstrated that, although the 19th
century excavator used very advanced techniques, there are still a lot
of loose teeth left in his back dirt (Strauss et al. 1988).
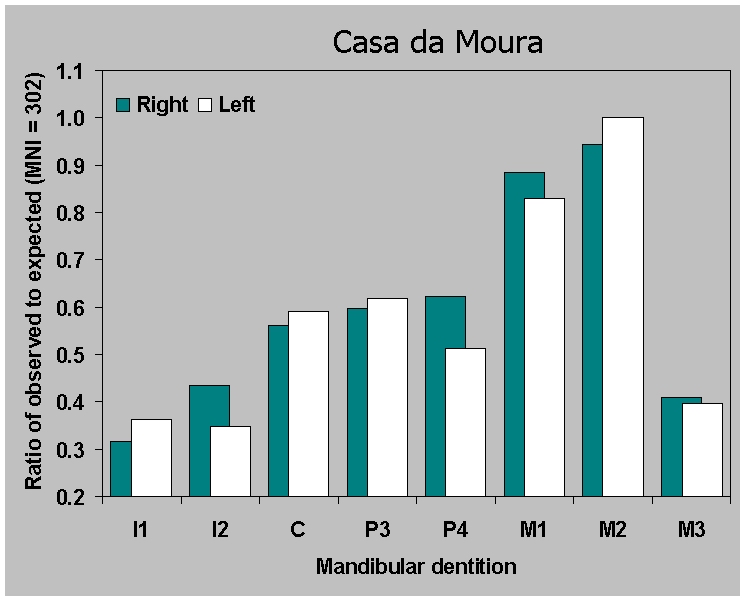
Figure 6a,b
shows a very specific pattern of adult tooth preservation (ignoring the
question of premortem tooth loss). Since the right and left sides are
almost identical, we know that a specific taphonomic factor, not
chance, is at work here. It is striking how similar the curves are for
the right and left sides considering when the bones were excavated and
how they have been stored, let alone the enormous problems of
identification of thousands of loose teeth.
Besides the comparable preservation of sides, what other evidence
suggests that there is any general significance to the pattern of
preservation? Analyses of Portuguese Mesolithic burials and Neolithic
ossuaries by Meiklejohn and Jackes and work by Duarte (1993) on the
later Neolithic ossuary of Carenque, allow us to make comparisons among
sites. The two Mesolithic sites show a simple pattern: mandibular
dentition (Figure 7b) is better preserved
than maxillary (Figure 7a), and first,
followed by second molars are the most commonly preserved teeth, while
incisors and third molars are the least commonly preserved. The
Neolithic sites provide a similar picture for mandibles but the
maxillary dentition is more varied in its preservation.
First, there is no similarity among Neolithic sites in the percent
frequency of teeth shed from alveoli (Figure 8a,b). This could be due
to depositional or
cultural factors or to excavation and storage techniques. We cannot
make definite statements about which teeth are always more likely to
fall out of their sockets and be found loose in deposits, but we can
predict that these loose teeth are likely to be maxillary teeth,
especially incisors and canines, followed in some sites by mandibular
incisors and maxillary premolars.
In general, mandibles (Figure 8b) are
more likely than maxillae to retain teeth in the bone (Figure 8a). That
is to be expected.
Nineteenth century French palaeontologists had already noted the
differential survival of upper and lower jaws and the same observation
has been made by many modern faunal analysts. Tobias (1987) has used it
as a measure of severity of taphonomical effects on East African
hominid samples.
We would also have expected complex multiple rooted teeth to be more
secure, and generally the first molar, especially on the mandible, is
retained in the bone. However, it is unexpected that 65% to 70% of all
Casa da Moura mandibular teeth samples are loose: we did not expect
that there is really very little difference across tooth types on Casa
da Moura mandibles.
While the retention of teeth in the bone is one factor to be
considered, the second factor is differential preservation of teeth
(Figure 9). The ratio of observed to expected is similar for maxillae
(Figure
9a) across sites. But for the
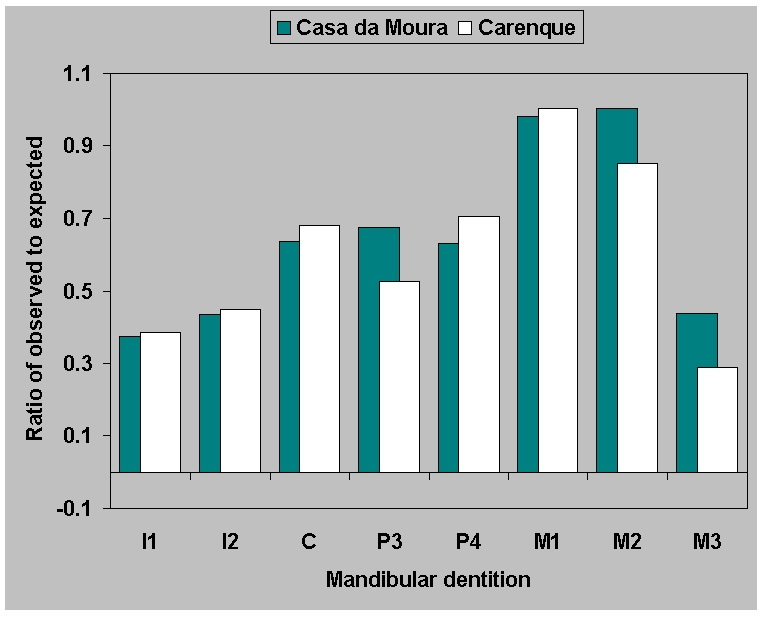
mandibles (Figure 9b) the ratio of
observed to expected is virtually identical in the two Neolithic
ossuaries. This is surprising, considering all the factors which could
bias the samples, including the difficulty of excavating and
identifying thousands of loose teeth. The pattern is illustrated
(Figure 10) by the Casa da Moura data,
including premortem tooth loss along with surviving teeth. We can
assume that we are saying something real here about preservation of
mandibular dentition. Since a broadly similar pattern also occurs in
the Mesolithic individual burials, which are a thousand years older,
this is a justifiable assumption.
Cultural factors during life may intrude. For example, the premortem
tooth loss rate for Casa da Moura right central lower incisors is 64%.
This is extraordinarily high and is no doubt due to the use of anterior
teeth as tools (identified in all Portuguese Mesolithic and Neolithic
samples we have examined; see also Lefèvre 1973 and
Fléchier et al. 1976).
Cultural practices at burial could also account for some variations
in the representation of teeth. We need not go as far as the 19th
century excavator of Casa da Moura who speculated on
cannibalism (Delgado 1867), but the point must be made that we do not
know the burial rites
involved.
Cultural practices may be a factor, but it seems more likely that we
can discern some general facts
about the differential survival of teeth. There is no strong
correlation between representation and
percent retained in the bone ( maxillae r = .230; mandibles r = .069:
based on data from Casa da
Moura, Carenque and the incompletely excavated ossuary of Feteira;
Zilhão 1984), but in both
cases mandibular cheek teeth are most likely to be at the high end of
the scale. Those most likely
to survive are mandibular first and second molars (though strong rooted
upper central incisors and
upper canines may also be well represented in ossuaries). The teeth
most likely to be retained in
alveoli are mandibular, especially mandibular cheek teeth.
If we can take this as evidence of a common pattern of differential
preservation of teeth in archaeological sites, then it has a bearing on
our methods of reporting rates of pathology.
We have questioned our ability to provide "real, meaningful" dental
pathology rates because of problems of observing and identifying
caries, especially at the cemento-enamel junction. Now we want to
question whether archaeological samples can ever provide accurate
incidences in age-dependent characters like dental pathology.
Different tooth classes have different susceptibility to pathology.
The complex topography and grinding function of molars and premolars
increases the likelihood of caries over that in the simple, cutting
anterior dentition (incisors and canines). If tooth type sample sizes
are unequal, then the overall pathology rates will be biased. If there
are more molars and fewer anterior teeth in the sample, reported
pathology rates will be higher than actually occurred in the living
population. And that must be the case with the Portuguese ossuaries. In
fact, Casa da Moura had quite low pathology rates. If we calculate a
caries rate from our preliminary work using all teeth, the overall
mandibular caries rate is around 6%, but the real caries rate must have
been lower, simply because the teeth which are least likely to have
caries are the teeth which are least likely to be represented in the
sample.
Because of this all our work on Portuguese dental pathology is based
only on one tooth class - lower molars (Jackes 1988; Lubell and Jackes
1985, 1988; Jackes et al. 1991; Lubell et al.
1994). It is quite clear that our chosen method is flawed because in
every site there is a bias against lower third molars, too great to be
explained by slow eruption or agenesis of third molars.
The more important question is whether the age distribution of the
preserved tooth types differs, and we will search for an answer using
mandibular canines, premolars and second molars, all of which have more
or less equivalent eruption times. The question is relevant because our
analyses show that mandibular canines and premolars may be
underrepresented in Neolithic samples by up to 40%, despite relatively
high frequencies of retention in alveoli (nearly 40%).
In Figure 11 the four teeth are plotted
as cumulative percentages of the minimum number of individuals (MNI )
of 302 individuals aged about 11 and over. This MNI is derived
separately from first and second molars and from right and left sides,
so we can be fairly certain that it is a good estimate of adolescent
and adult population size. To the samples of teeth in the older age
category, we add the teeth we know were lost premortem.
Figure 11 raises the question of how
second molar wear is related to
wear on teeth more anterior in the jaw. It is most probable that the
attrition grades of Smith (1984), which we used as the starting point
for our work, lump too many molars into wear level 3. For more detailed
work we use finer gradations. But leaving aside the need for a little
smoothing of the M2 curve, we see that the M2 sample is least biased.
The premolar and canine samples represent less than 60% of the
population. Granted the deficiencies of the wear grading system, we can
still say that the inequalities in the samples are most evident in
middle and late adulthood. What does this inequality do to the caries
rate?
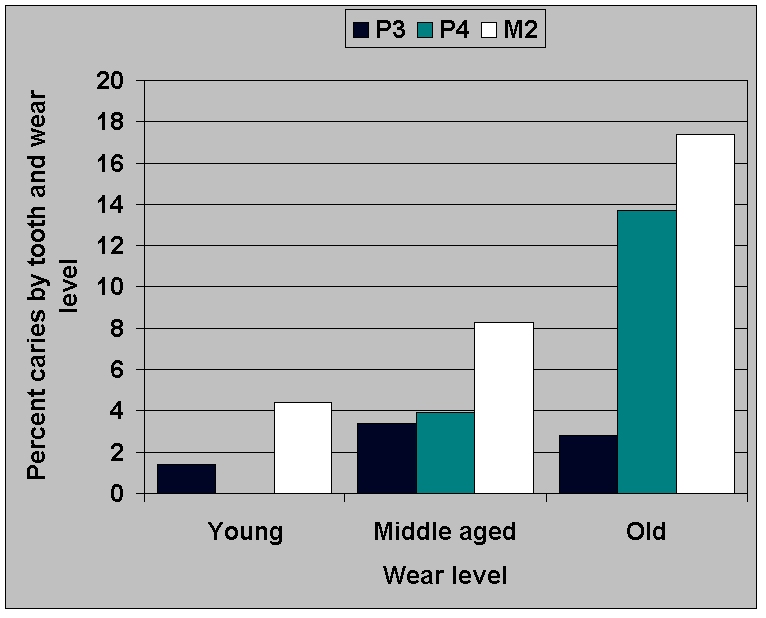
In Figure 12 the premolars and second
molars are grouped into three
age classes:
1) adolescent and young adult from eruption to the point where no
more than a pinpoint of dentin shows;
2) a very general class which includes a broad range of adults from
age 30 on through middle age: canine cemental annulation analysis
suggests up to 55 years;
3) a third class which, in molars, comprises all those cases in
which the fissures have been completely obliterated and the dentin
exposures are coalescing: in canines cemental annulation analysis
suggests ages 55 to around 80.
Canines are absent from Figure 12
because lower canines were free of
caries. But there is premortem canine loss, some of it probably
attributable to caries for the reason that a third of Casa da Moura
lower first premolar caries are mesial interproximal.
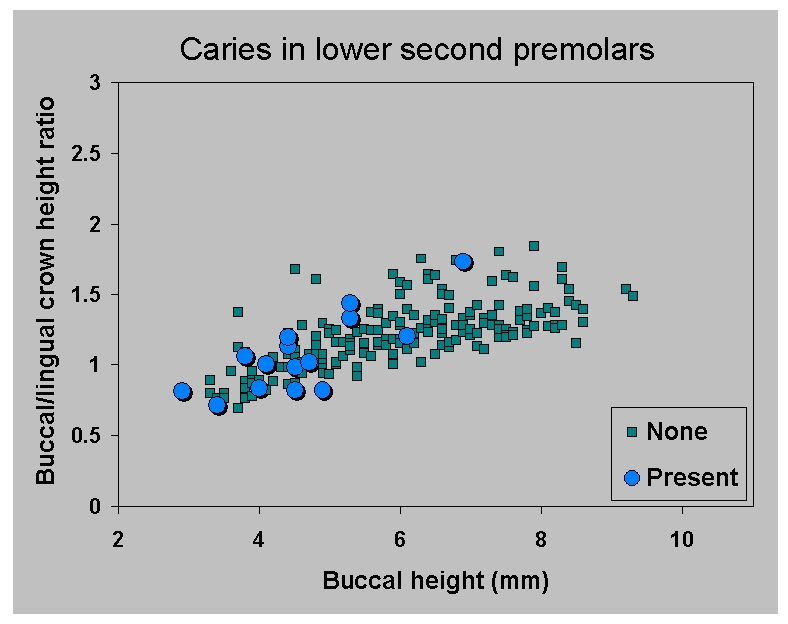
Caries rates increase into old age as expected in the second molar
(M2) and the second premolar (P4). But in the first premolar (P3)
caries rates actually decrease into old age. Since the two premolar
teeth experience equivalent premortem tooth loss (12% in P3 vs. 15% in
P4), and nearly half the lower second premolar caries are mesial, it is
possible that carious first premolars in higher wear categories are
underrepresented. Furthermore, first premolars in higher wear
categories have higher frequencies of CEJ erosion than do second
premolars (7% vs. 6% strongly marked erosion on the preliminary
analysis).
Analysis of the distribution of first
and second premolars over the
three broad wear categories shows no significant difference (chi square
P = .164), indicating that errors in coding for wear do not explain the
difference between the two teeth. Nevertheless, this possibility must
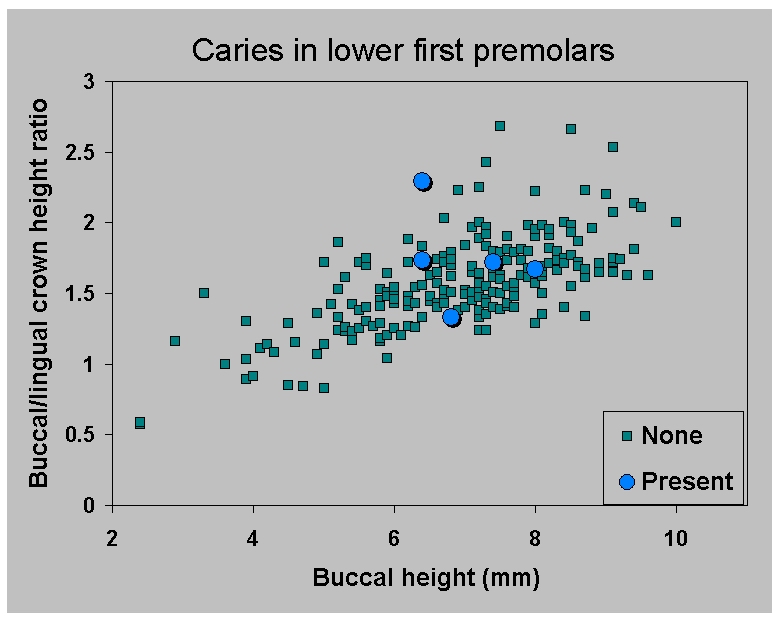
be controlled for. Figure 13a confirms
that third premolars do indeed
lack caries in older age categories, as determined by objective
measurement of buccal crown height and the ratio of buccal to lingual
crown height, not by subjective wear assessments. The value of these
two characteristics was determined on the basis of discriminant
function analysis which showed them to discriminate among the grouped
wear levels. Buccal height contributes most strongly to function 1
which explains 98-99% of the variance.
The combined effects of carious first premolar underrepresentation
in older age categories, and the masking effect of the CEJ erosion,
means that we cannot know the caries rate for first premolars. The
underrepresentation of lower canines, together with a rate of CEJ
erosion even higher than for the lower first premolars (8% marked
erosion vs. 7% for P3), again means that we have no clear idea at all
of the caries rate in lower canines.
Conclusion
There is differential tooth preservation in archaeological sites.
Since pathology is age-dependent, the combined effect of the
preferential loss of older teeth in categories that suffer least dental
pathology means that we are truely unable to estimate overall dental
caries rates.
Combined with other considerations including the unknown age
distribution of the dead and various problems of defining and observing
caries which will differ among sites, our chances of correctly
assessing changes in caries rates through time and correctly
attributing all such changes to diet alone are not high.
Acknowledgements
The research for this paper was made possible by Grants 810-84-0030
and 410-86-2017 from the Social Sciences and Humanities Research
Council of Canada to DL, MJ and Christopher Meiklejohn and additional
grants to DL from the Central Research Fund, University of Alberta.
Research on taphonomy by MJ in East Africa (made possible through a
grant from the L.S.B. Leakey Foundation, but unpublished because the
data were in baggage lost by PanAmerican Airlines in 1976) provided the
impetus for the ideas expressed here. MJ thanks Susan Pfeiffer for many
years of discussions on the interpretation of ossuaries. We are
grateful to Dr. C. Meiklejohn for generously sharing his data, and
especially to Dr. M.M. Ramalho of the Geological Survey of Portugal,
Lisbon, for allowing us to work on the magnificent collections of
skeletal remains under his care and for facilitating our research. The
work of several students has been important to our research: G.S. Tait,
Cidália Duarte and especially Pamela Mayne. Previous versions of
this paper have been given as Jackes et al., 1991 and Jackes
1992.
References
Delgado, J.F.N. (1867). Da existência provável do
homem no nosso solo em tempos mui remotos provada pelo estudo das
cavernas. I -- Noticia acerca das grutas da Cesareda. Estudos
Geologicos, Commissão Geologica de Portugal, Lisboa.
Duarte, C.M.P. (1993). Analysis of wear and pathological
conditions in human teeth from the Neolithic site of Grutas Artificias
do Tojal de Vila Chã, Carenque (Estremadura, Portugal). MA
Thesis, Department of Anthropology, University of Alberta.
Fléchier, J.-P., Lefèvre, J. and Verdene, J. (1976).
Mensurations dentaires des hommes de Muge. Bulletins et
Mémoires de la Société d'Anthropologie de Paris
t.3, série XII: 147-164.
Frayer, D. (1987). Caries and oral pathologies at the Mesolithic
sites of Muge: Cabeço da Arruda and Moita do Sebastião. Trabalhos
de Antropologia e Etnologia (Porto) 27: 9-25.
Fure, S. and Zickert, I. (1990). Root surface caries and associated
factors. Scandinavian Journal of Dental Research 98: 391-400.
Jackes, M. (1988) The Osteology of the Grimsby Site.
Edmonton: Department of Anthropology, University of Alberta.
Jackes, M. (1990). Diagenetic change in prehistoric Portuguese
human bone (4000 to 8000 BP). Paper presented at the 18th Meeting,
Canadian Association for Physical Anthropology, Banff, Alberta.
Jackes, M. (1992). Taphonomy and the human osteologist. Paper
presented at the 20th Meeting, Canadian Association for Physical
Anthropology, Edmonton, Alberta.
Jackes, M., Lubell, D. & Meiklejohn, C. (1991). Will the real
caries rate please identify itself? Paper presented at the 19th
Meeting, Canadian Association for Physical Anthropology, Hamilton,
Ontario.
Jackes, M., Barker, C.M. and Wayman, C.L. (1992) Bacterial effects
on human bone from archaeological sites. Paper presented at the 20th
Meeting, Canadian Association for Physical Anthropology, Edmonton,
Alberta.
Jackes M., Sherburne R., Lubell
D., Barker C. & Wayman M. 2001 Destruction of
microstructure in
archaeological bone: a case study from Portugal. International Journal of Osteoarchaeology
11: 415-432.
Lefèvre, J. (1973). Etude odontologique des hommes de Muge. Bulletins
et Mémoires de la Société d'Anthropologie de Paris
10: 301-333.
Lubell, D. & Jackes, M. (1985). Mesolithic-Neolithic
continuity: evidence from chronology and human biology. In (M. Ramos,
Ed) Actas, I Renunião do Quaternário Iberico
(Lisboa, 1985), pp. 113-133.
Lubell, D. & Jackes, M. (1988). Portuguese Mesolithic-Neolithic
subsistence and settlement. Rivista di Antropologia (Roma),
Supplemento del Vol. LXVI: 231-248.
Lubell, D., Jackes M., Schwarcz, H., Knyf, M. and Meiklejohn, C.
(1994). The Mesolithic-Neolithic transition in Portugal: isotopic and
dental evidence of diet. Journal of Archaeological Science
21(2): 201-216.
Martin R.B. and Burr D.B. (1989). Structure, Function, and
Adaptation of Compact Bone. New York: Raven Press.
Masset, C. (1973). Influence du sexe et de l'âge sur la
conservation des os humains. In Sauter M (ed): L'Homme, Hier et
Aujourd'hui: Recueil d'Études en Hommage à André
Leroi-Gourhan. Paris: Cujas, pp 333-343.
Mazess, R.B. (1983). Noninvasive bone measurements. Skeletal
Research 2: 277-343.
O'Sullivan, E.A., Williams, S.A., Wakefield, R.C., Cape, J.E. and
Curzon, M.E.J. (1993). Prevalence and site characteristics of dental
caries in primary molar teeth from prehistoric times to the 18th
Century in England. Caries Research 27: 147-153.
Schwarcz, H.P., Melbye, J. & Katzenberg, M.A. (1985). Stable
isotopes in human skeletons of
southern Ontario: reconstructing paleodiet. Journal of
Archaeological Science 12:
187-206.
Smith B.H. (1984). Patterns of molar wear in hunter-gatherers and
agriculturalists. American Journal of Physical Anthropology
63: 39-56.
Straus, L.G., Altuna, J., Carvalho, E., Jackes, M. & Kunst, M.
(1988). New excavations in Casa da Moura (Serra d'el Rei, Peniche) and
at the Abrigos de Bocas (Rio Maior), Portugal. Arqueologia
18: 65-95.
Tobias, P. (1987). On the relative frequencies of hominid maxillary
and mandibular teeth and jaws as taphonomic indicators. Human
Evolution 2: 297-309.
Toussaint, M. (1991). Étude spatiale et taphonomique de deux
sépultures collectives du néolithique récent:
l'Abri Masson et la Fissure Jacques à Sprimont, Province de
Liège, Belgique. L'Anthropologie 95: 257-278.
Zilhão, J. (1984). A Gruta da Feteira
(Lourinhã): Escavação de salvamento de uma
necrópole neolítica. Lisboa: Trabalhos de
Arqueologia 01.
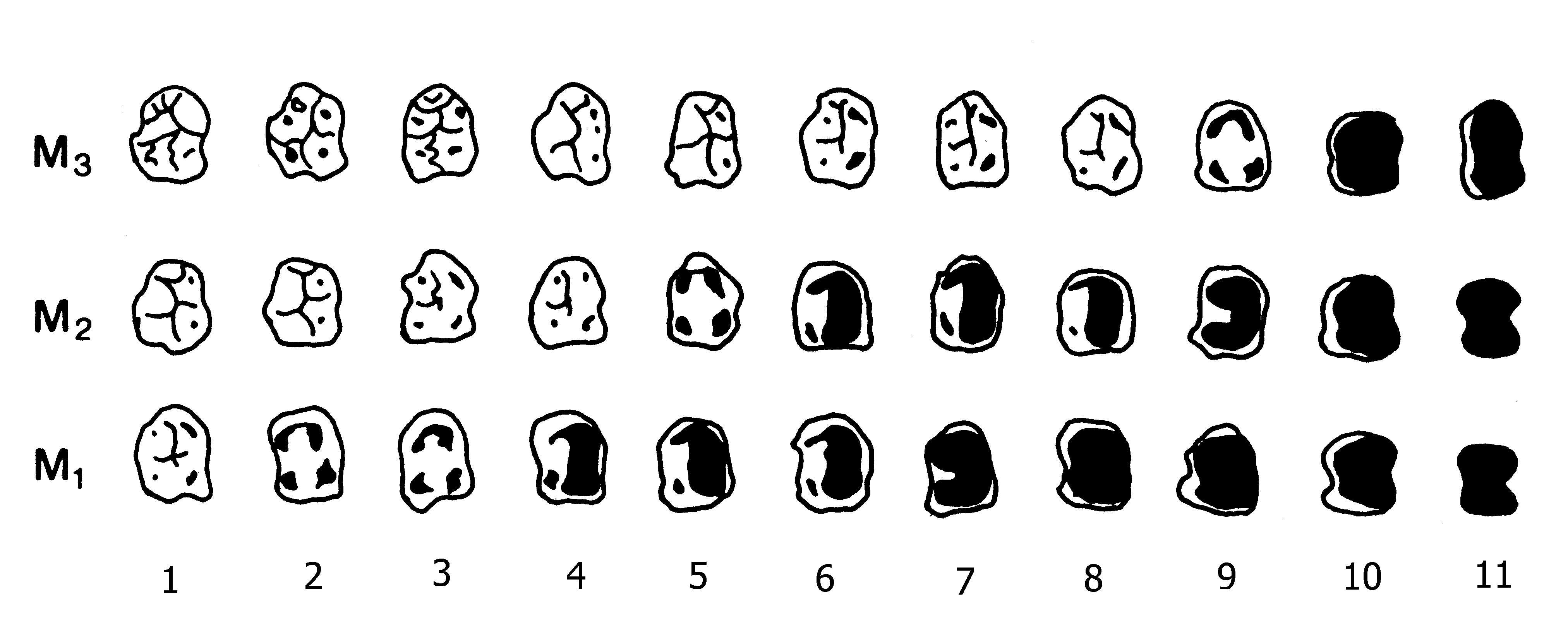
Figure 1.
Caries rates for Mesolithic Portuguese samples from
Cabeço da Arruda and Moita do Sebastião as recorded by
different investigators using individuals (Sueiro), all teeth
(Ferembach, Meiklejohn 1, Frayer), lower molars only (Jackes), molars
and premolars (Meiklejohn 2)
|
|
Figure 2.
Cabeço da Amoreira 7, as preserved in the museum of
the Serviços Geológicos in Lisbon, showing partially
cleaned matrix obscuring interproximal surfaces.
|
|
Figure 3.
Changes through time in mandibular molar
pathology in the ratio of interproximal to occlusal caries. Calibrated
mean radiocarbon years are shown.
|
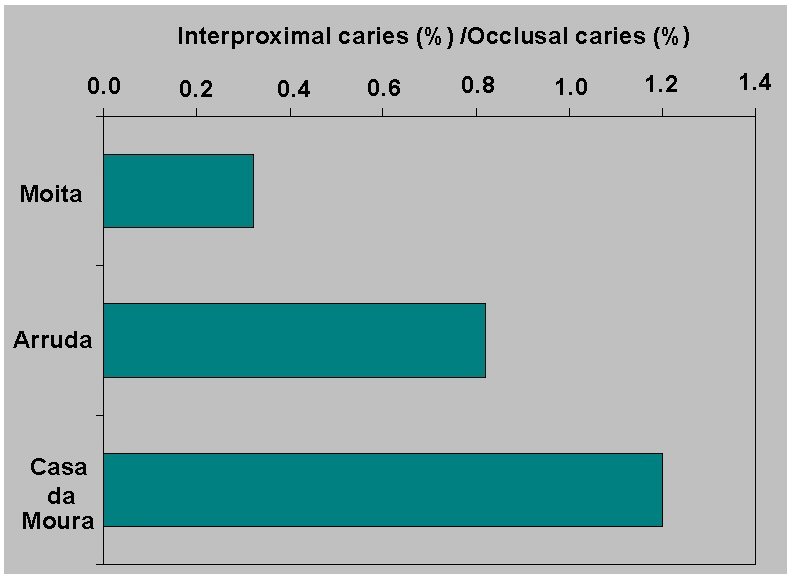
|
Figure 4a.
Caries and erosion at the
cemento-enamel junction, all specimens from Casa da Moura.
(a)
toothpick groove on a non-carious upper molar;
|

|
Figure 4b.
Caries and erosion at the
cemento-enamel junction, all specimens from Casa da Moura.
(b) caries on an upper
molar, note smooth radical margin;
|
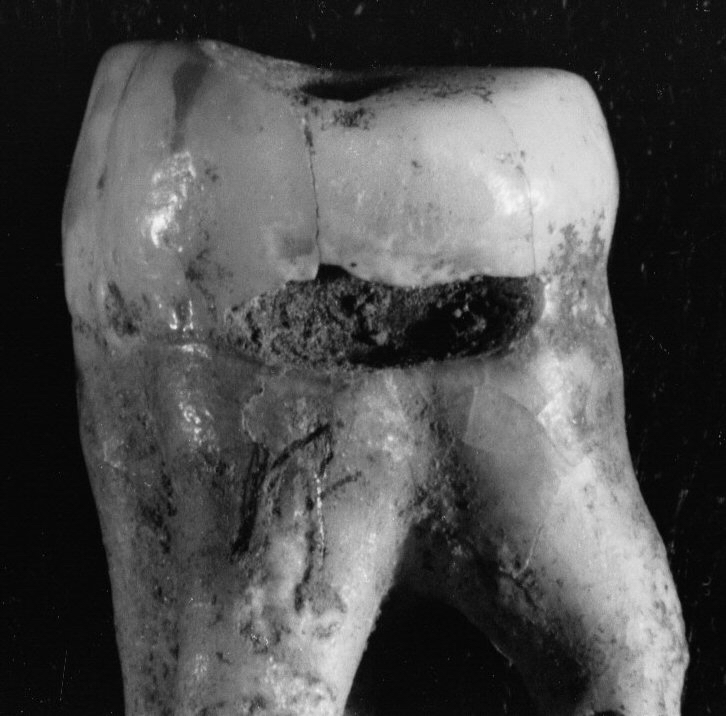
|
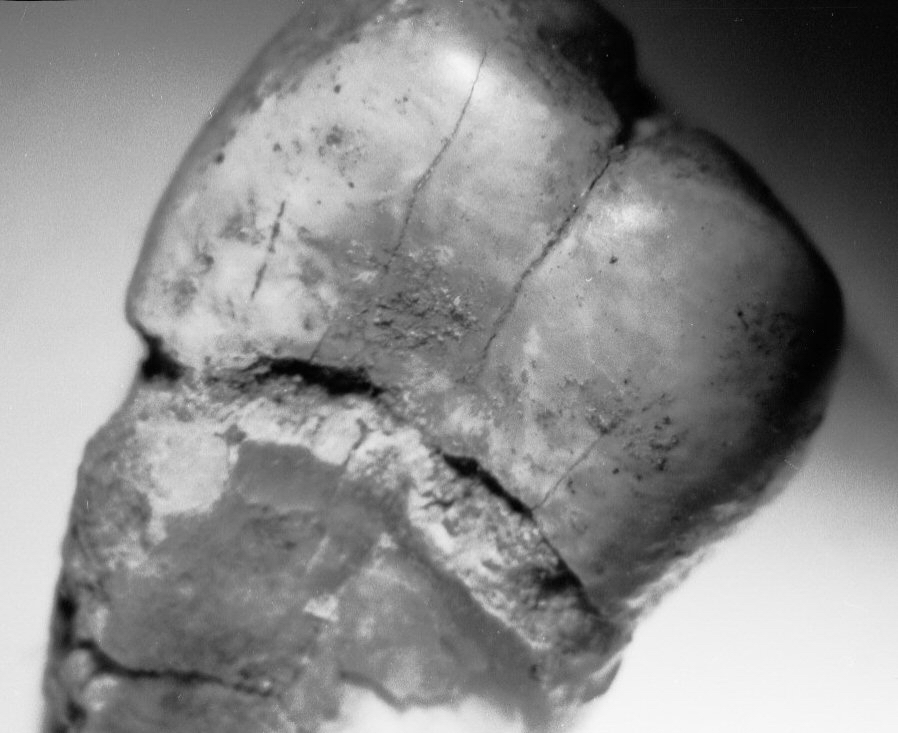
Figure 4c.
Caries and erosion at the
cemento-enamel junction, all specimens from Casa da Moura.
(c) post-mortem erosion on an upper
premolar at 6x;
|
|
Figure 4d.
Caries and erosion at the
cemento-enamel junction, all specimens from Casa da Moura.
(d) same specimen at 12x, note rough floor;
|

|
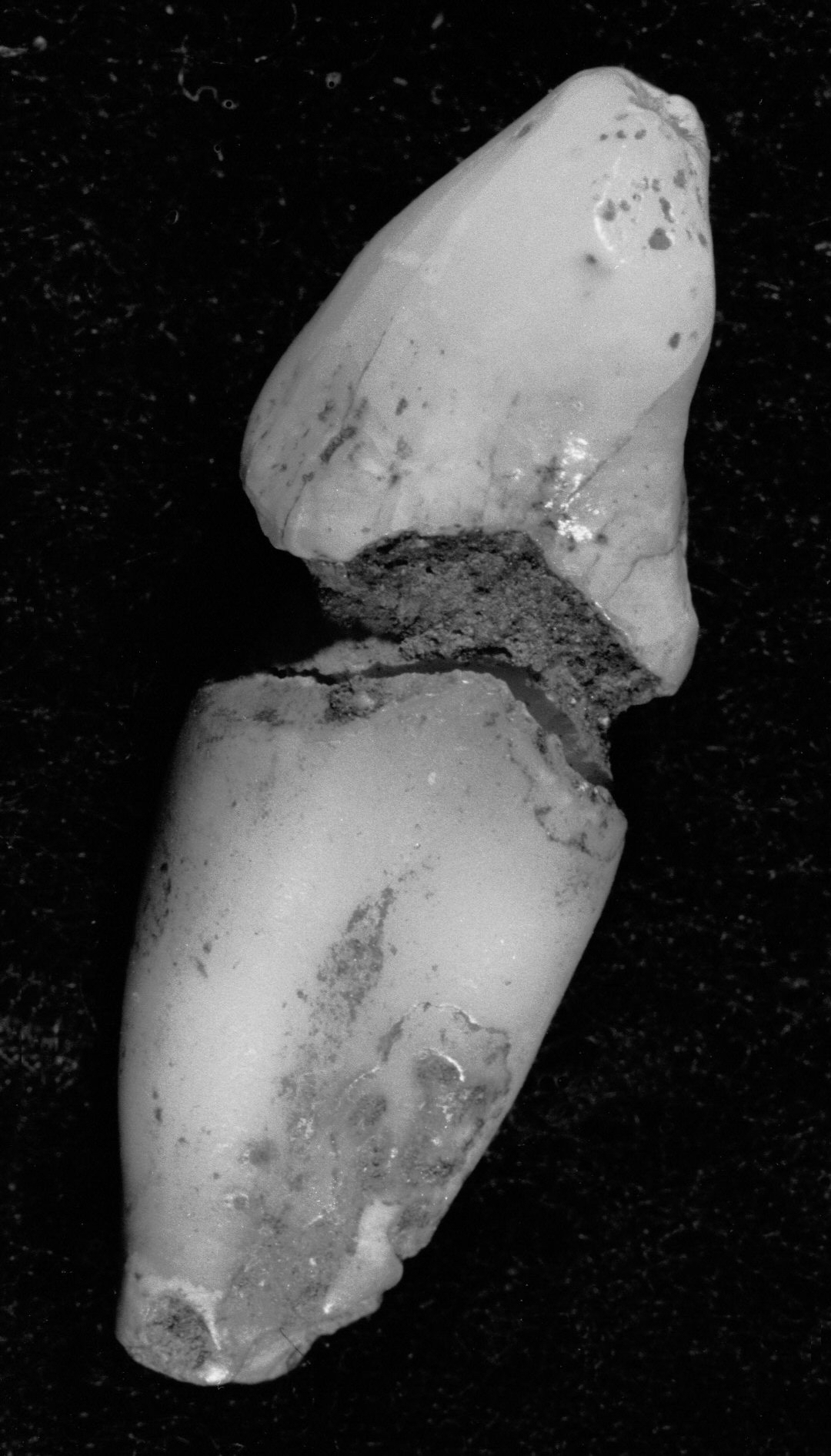
Figure 4e.
Caries and erosion at the
cemento-enamel junction, all specimens from Casa da Moura.
(e) extreme
grooving on a lower canine.
|
|
Figure 5a.
Comparison of the pathology rates
(% premortem tooth loss plus caries) within each attrition grade for
(a) Moita do
Sebastião. Shaded bars represent the
percent distribution of mandibles over 15 years of age across the 11
attrition grades (see Figure 5c).
|
|
Figure 5b.
Comparison of the pathology rates
(% premortem tooth loss plus caries) within each attrition grade for
(b) Cabeço da Arruda. Shaded bars represent the
percent distribution of mandibles over 15 years of age across the 11
attrition grades (see Figure 5c).
|
|
Figure 5c.
Moita do
Sebastião and Cabeço da Arruda mandibles over
15 years of age were distributed across the 11
attrition grades.
|
|
Figure 6a.
Ratio of observed to expected teeth for
(a) maxillary dentition from Casa da Moura based on
an MNI of 302 adolescents and adults.
|
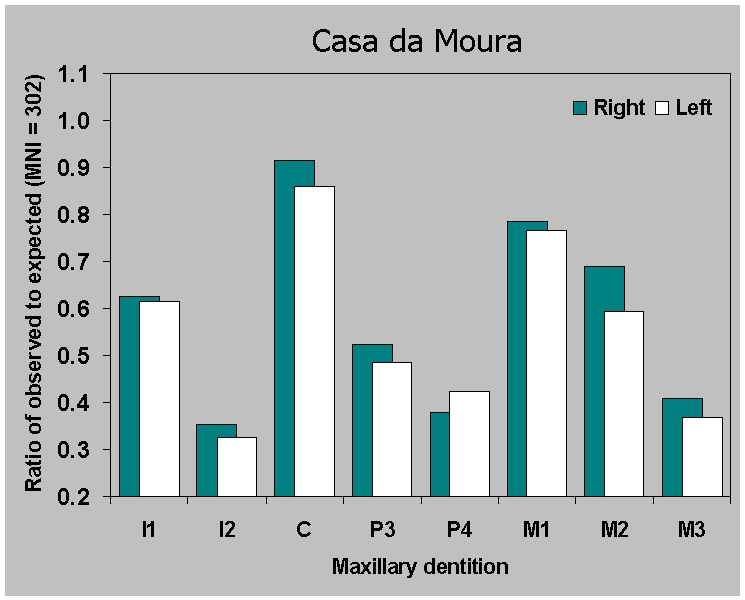
|
Figure 6b.
Ratio of observed to expected
teeth for (b) mandibular dentition from Casa da Moura based on
an MNI of 302 adolescents and adults.
|
|
Figure 7a.
Ratio of observed to expected
teeth for
(a) maxillary dentition from Moita do
Sebastião and Cabeço da Arruda based the number lower
first molars which are the most frequently preserved teeth.
|
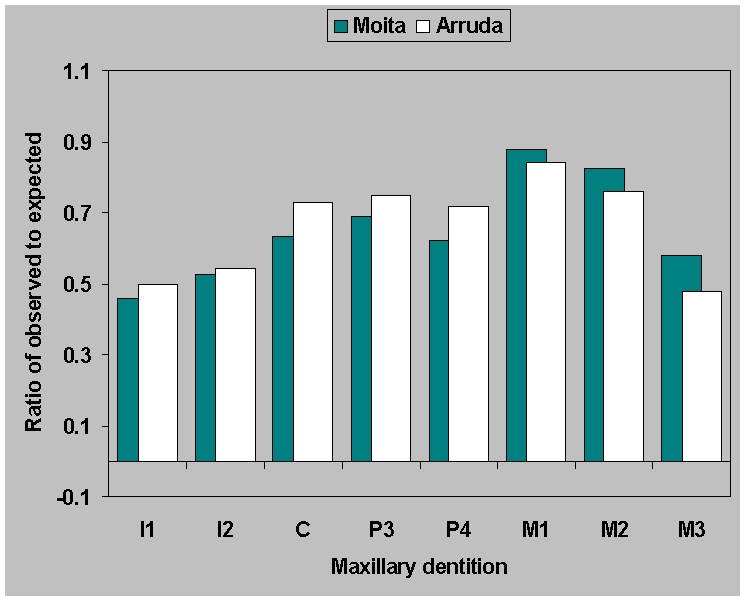
|
Figure 7b.
Ratio of observed to expected
teeth for (b) mandibular dentition from Moita do
Sebastião and Cabeço da Arruda based the number lower
first molars which are the most frequently preserved teeth.
|
|
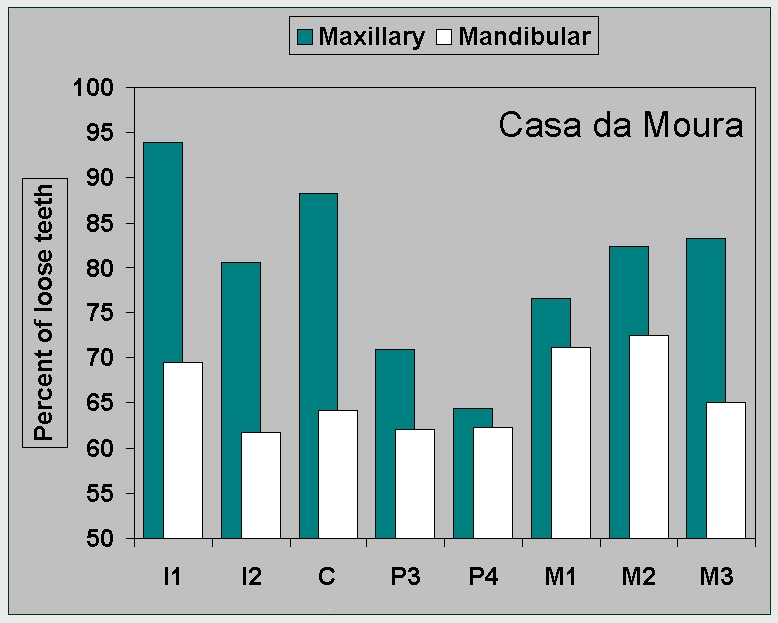
Figure 8a.
Percent of loose teeth in the
Neolithic
samples from (a) Casa da Moura.
|
|
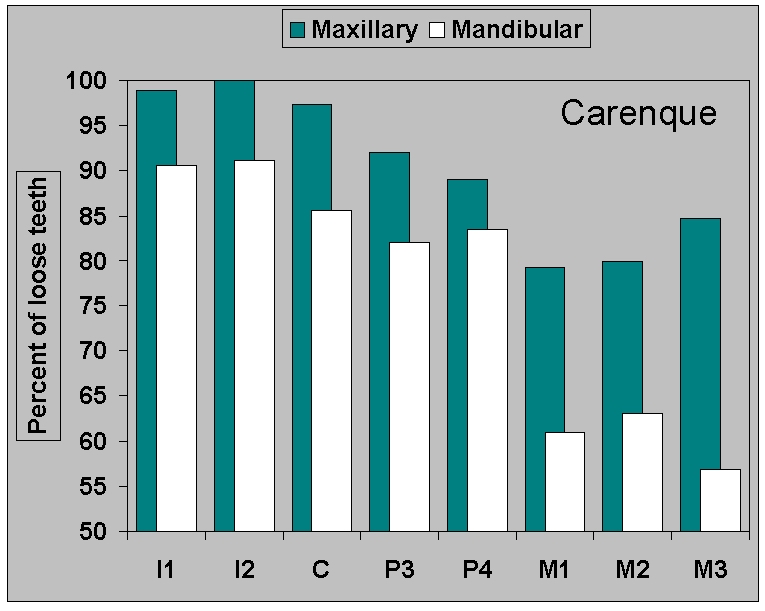
Figure 8b.
Percent of loose teeth in the
Neolithic
samples from (b) Carenque.
|
|
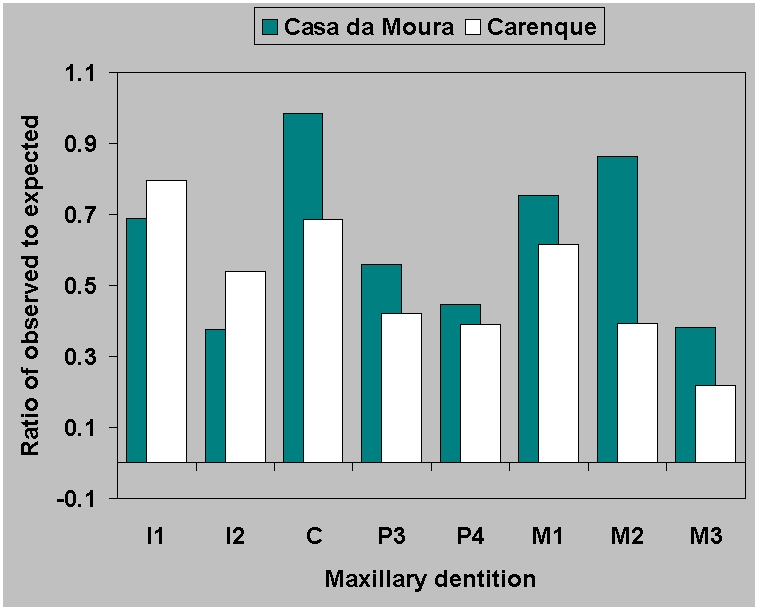
Figure 9a.
Ratio of observed to expected
teeth for
(a) maxillary dentition from Casa da Moura and
Carenque.
|
|
Figure 9b.
Ratio of observed to expected
teeth for (b) mandibular dentition from Casa da Moura and
Carenque.
|
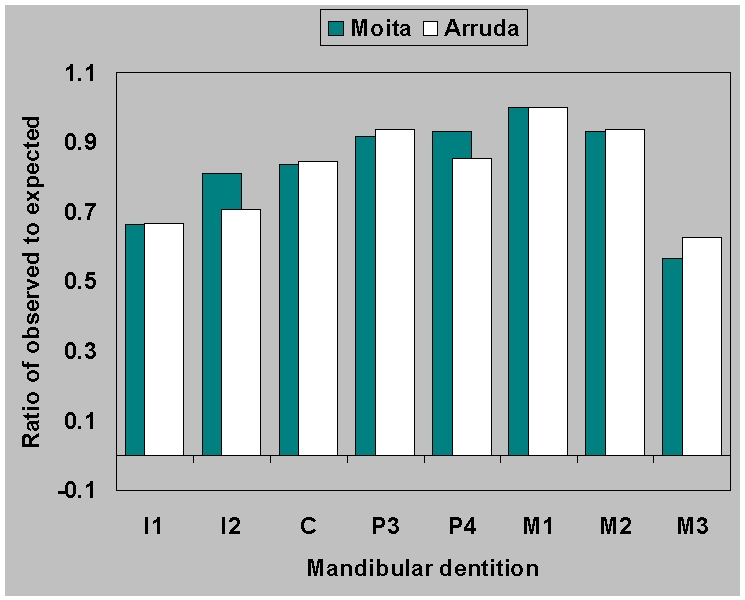
|
Figure 10.
Ratio of observed to expected teeth for
Casa da Moura loose mandibular teeth and mandibles with premortem tooth
loss included.
|
|
Figure 11.
Casa
da Moura: frequencies of
mandibular canines, premolars and second molars plotted as cumulative
percentages across attrition stages, premortem tooth loss included.
|
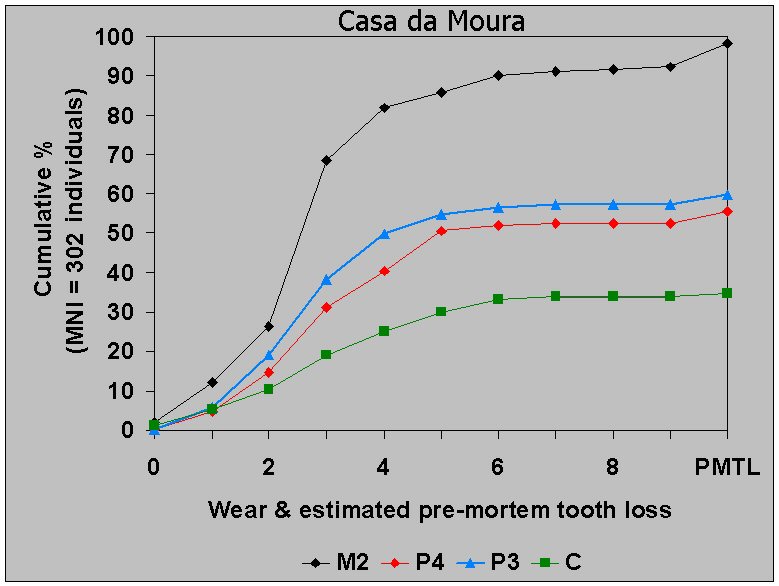 |
Figure 12.
Casa da Moura: frequencies of
caries in
mandibular premolars and second molars by grouped wear levels.
|
|
Figure 13a.
Casa da Moura: distribution of
caries
in (a) lower first premolars
based on buccal crown height and angle of wear as expressed by the
ratio of buccal to lingual crown
height. Sample includes only those teeth in which root formation is
complete (apart from closure of the
tip).
|
|
Figure 13b.
Casa da Moura: distribution of
caries
in (b) lower second premolars
based on buccal crown height and angle of wear as expressed by the
ratio of buccal to lingual crown
height. Sample includes only those teeth in which root formation is
complete (apart from closure of the
tip).
|
|